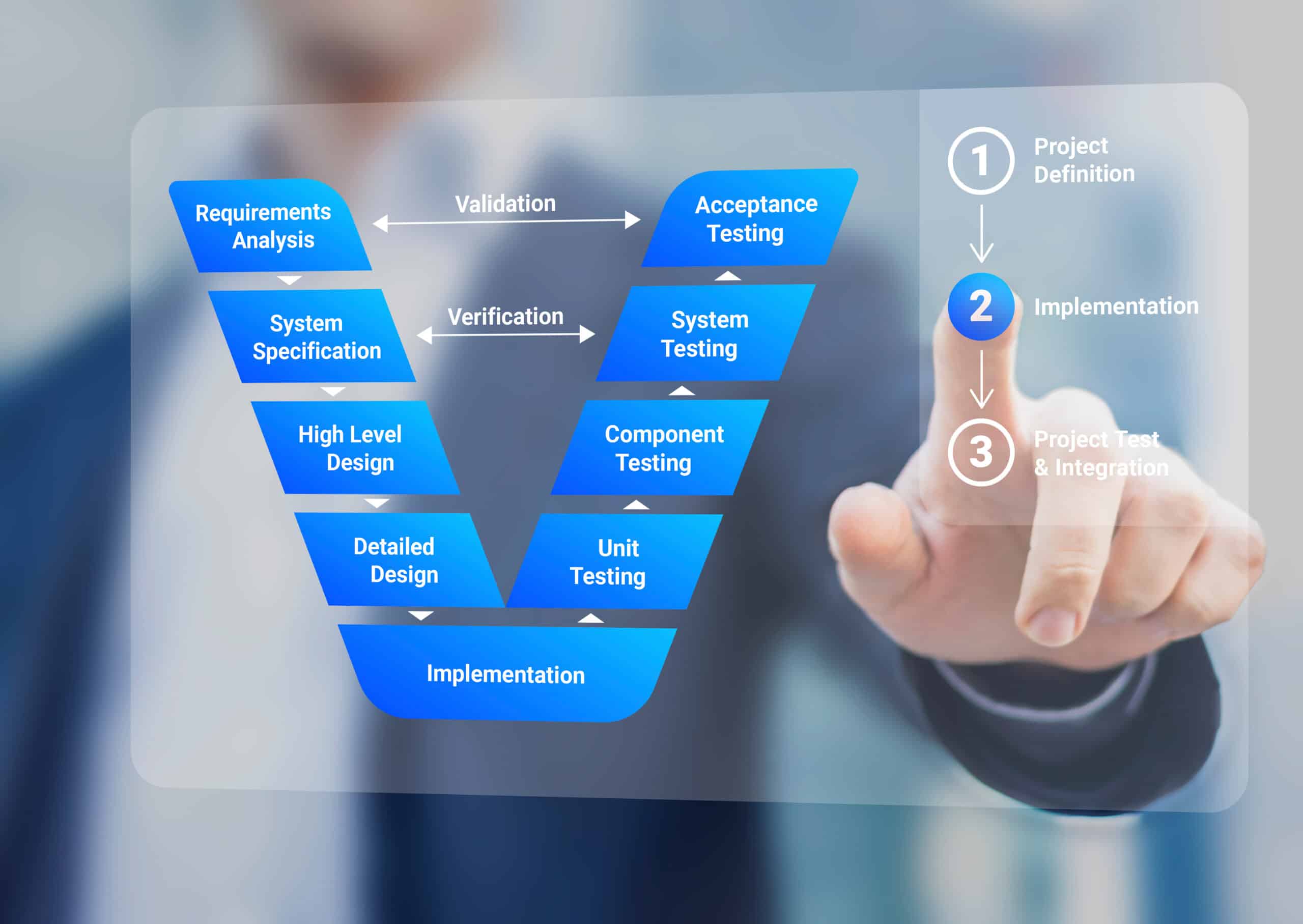
Computer Systems Validation (CSV) / Computer Software Assurance (CSA)
For the average business, managing the site quality validation function is a complex process to assure effective oversight of facility, equipment, utility, method, computerized systems, and process validation. At Xevalics, we follow our proven agile streamlined risk-based approach to make certain your site compliance with policies, procedures, and tools to ensure your regulated (GxP) systems are validated and controlled in accordance with the applicable US and EU GMP regulations and ICH guidelines. Our expert consultants have a thorough understanding of global regulations and industry-accepted software development standards.
With the new FDA CSA Guidance soon to be released, Xevalics will support your organization implementing CSA through agile project management, incremental adoption strategies, and strategic change management for an effective transition at your site. Xevalics consultants will help validate your equipment, systems, software, facilities, utilities, test methods, processes, and more. From remediation to facility expansions to quality initiatives, our team specializes in bringing solutions to complex problems across the pharmaceutical, biotech, and cell & gene therapy industries. Our team focus is on delivering excellence.
List of Expertise & Capabilities:
- Risk Assessments
- Validation Master Plans Development
- IQ/OQ/PQ Protocol Development and Execution
- 21CFR Part 11 and Annex 11 Compliance
- Computerized Systems Part 11 Gap Assessments
- Systems Retirement and Decommissioning
- Regulated Data Archival
- Third-Party Software Risk Assessments
- Business Continuity Plans
- Disaster Recovery Plans
- CSV Remediation
- Lab Instrument / Standalone System Qualification and Validation
Let’s Connect
Xevalics has the know how to skillfully manage projects, teams, and workload to support the validation lifecycle ensuring you get results faster with minimum impact to your business operations. We can assess your needs and implement solutions that ensure business continuity and faster results. Contact us today to discover how Xevalics can provide the support you need in completing your project.


